Enantioselective α-Vinylation of Aldehydes via the Synergistic Combination of Copper and Amine Catalysis
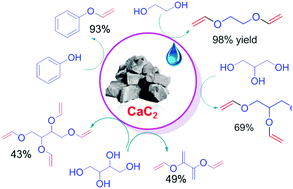
Direct vinylation of natural alcohols and derivatives with calcium carbide - Green Chemistry (RSC Publishing)
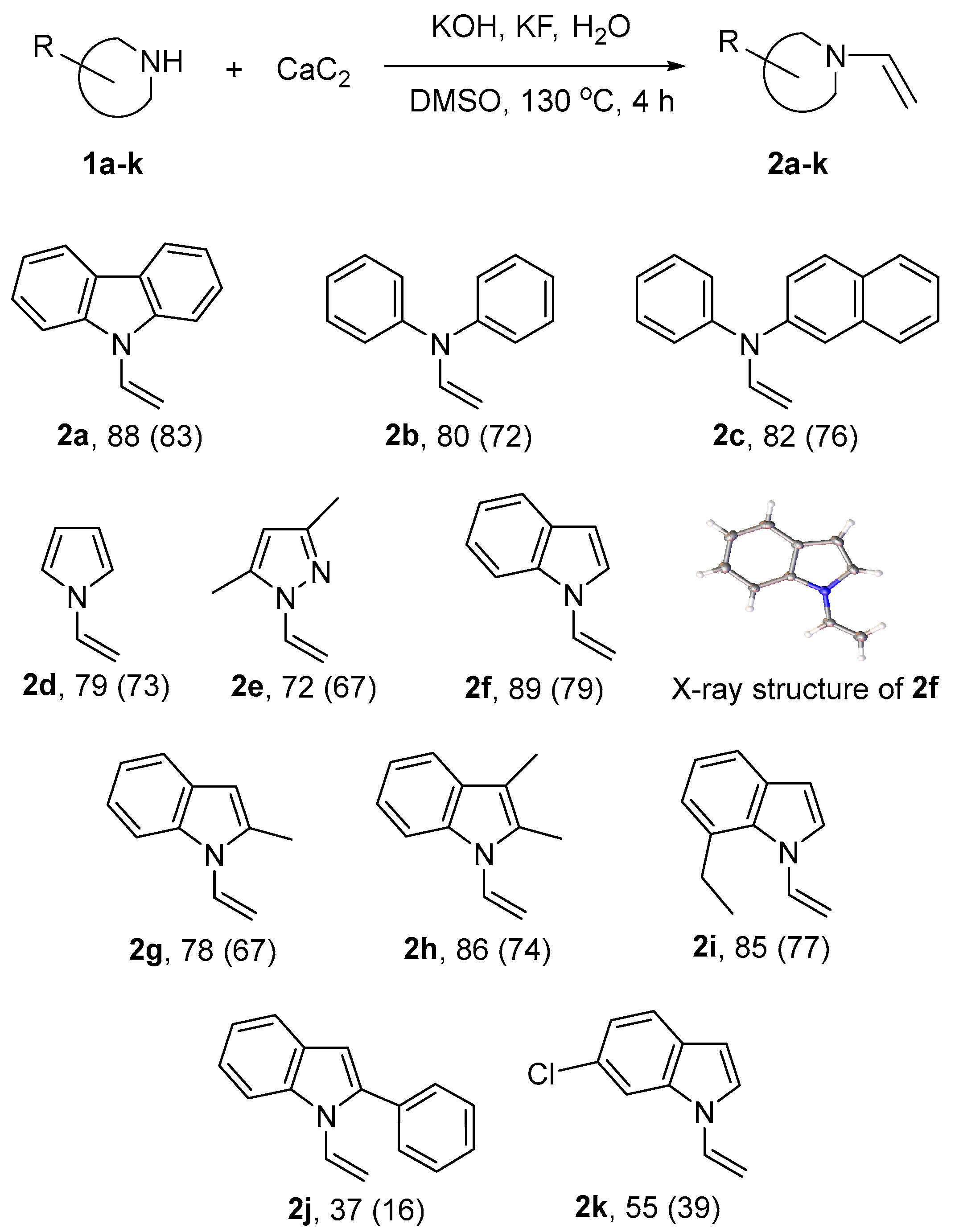
Molecules | Free Full-Text | Vinylation of a Secondary Amine Core with Calcium Carbide for Efficient Post-Modification and Access to Polymeric Materials

Vinylation of phenol by acetaldehyde: A new reaction for the synthesis of o-vinylphenol - ScienceDirect
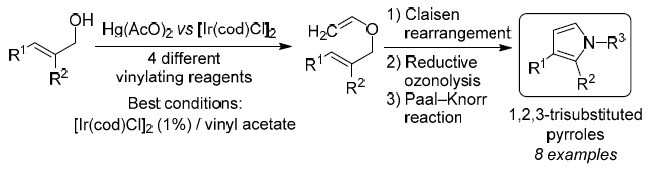
Synthesis of 1,3- and 1,2,3-functionalized pyrroles via Ir(I)-catalyzed vinylation of allyl alcohols | SpringerLink
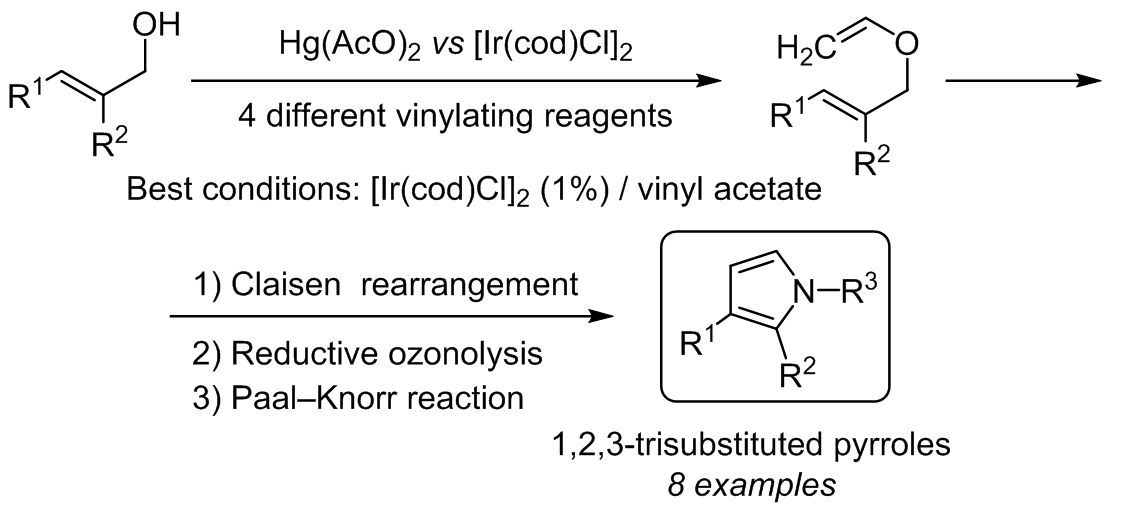
SYNTHESIS OF 1,3- AND 1,2,3-FUNCTIONALIZED PYRROLES <i>via</i> Ir(I)-CATALYZED VINYLATION OF ALLYL ALCOHOLS | Alavez-Rosas | Chemistry of Heterocyclic Compounds

Vinylation of aromatic halides using inexpensive organosilicon reagents. Illustration of design of experiment protocols. - Abstract - Europe PMC

Plausible mechanism for the vinylation of alcohols and phenols in the... | Download Scientific Diagram
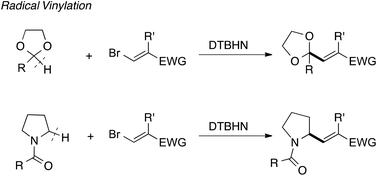
Radical vinylation of dioxolanes and N-acylpyrrolidines using vinyl bromides - Organic Chemistry Frontiers (RSC Publishing)
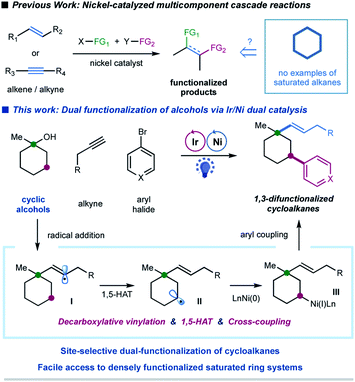
Sequential C–O decarboxylative vinylation/C–H arylation of cyclic oxalates via a nickel-catalyzed multicomponent radical cascade - Chemical Science (RSC Publishing) DOI:10.1039/D0SC01471K

Use of Iridium‐Catalyzed Transfer Vinylation for the Synthesis of Bio‐Based (bis)‐Vinyl Ethers - Spiegelberg - 2022 - Advanced Synthesis & Catalysis - Wiley Online Library
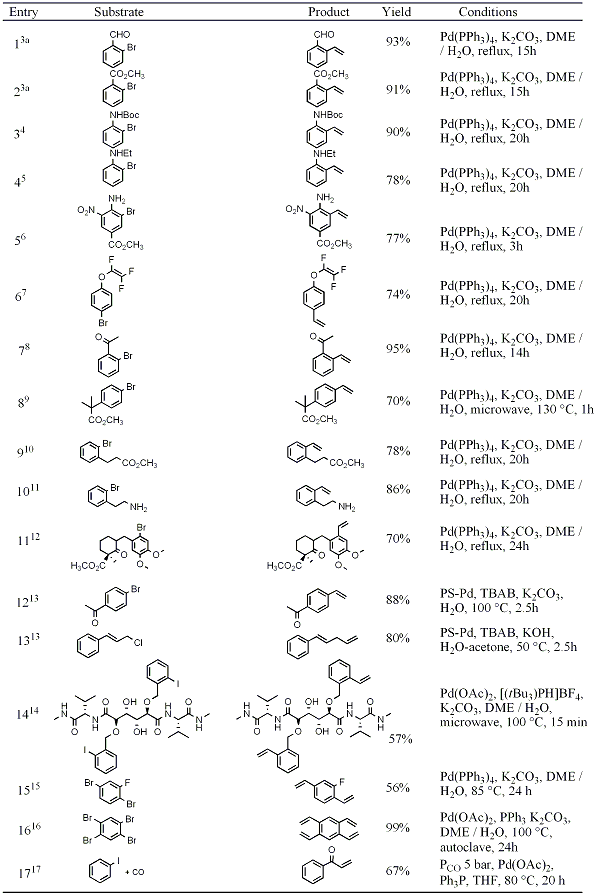
![PDF] Vinylation of a Secondary Amine Core with Calcium Carbide for Efficient Post-Modification and Access to Polymeric Materials | Semantic Scholar PDF] Vinylation of a Secondary Amine Core with Calcium Carbide for Efficient Post-Modification and Access to Polymeric Materials | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/f286ceb376de35cacaa09ad45f90c27e91883fe4/3-Table1-1.png)
PDF] Vinylation of a Secondary Amine Core with Calcium Carbide for Efficient Post-Modification and Access to Polymeric Materials | Semantic Scholar
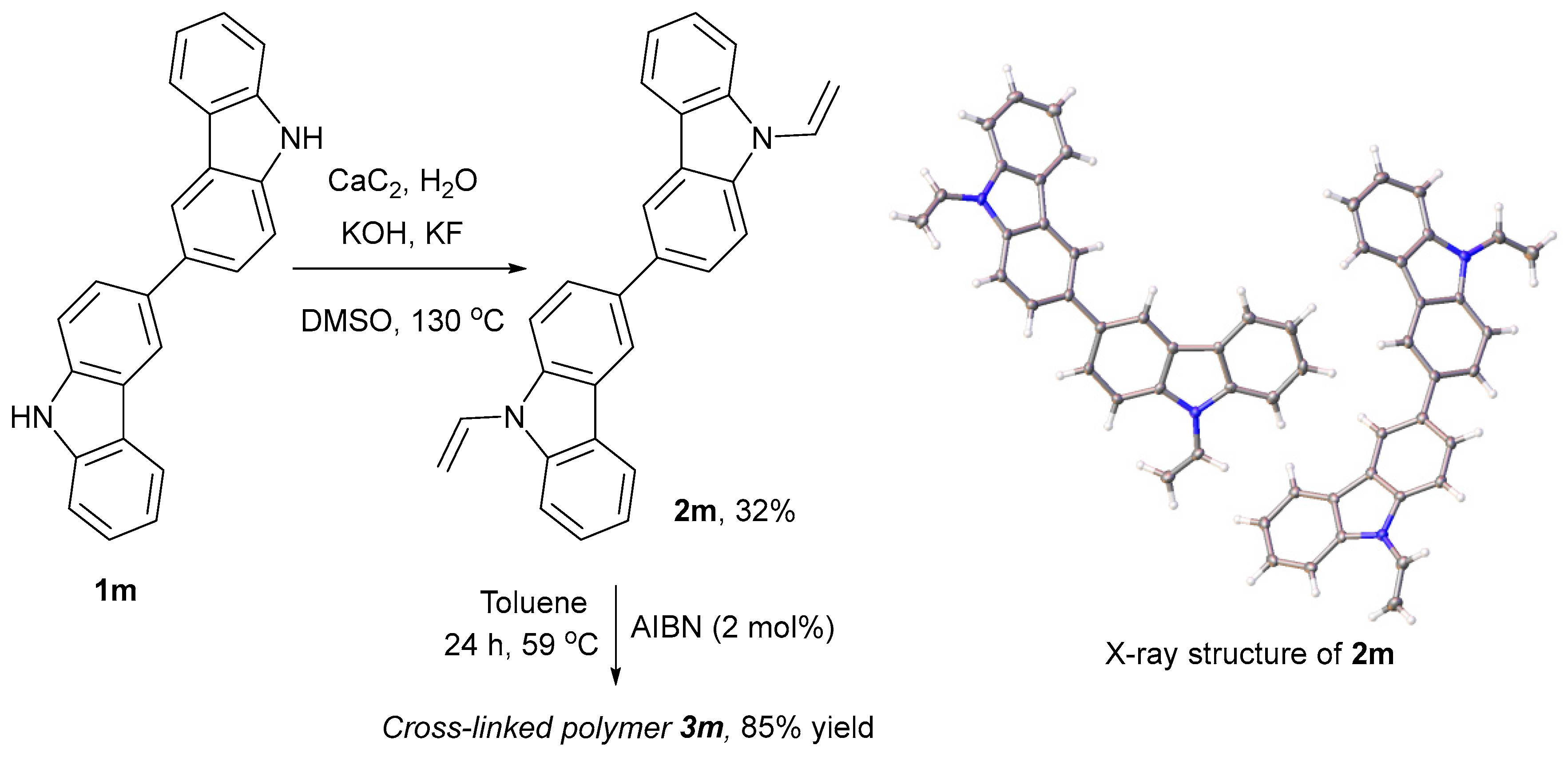
Molecules | Free Full-Text | Vinylation of a Secondary Amine Core with Calcium Carbide for Efficient Post-Modification and Access to Polymeric Materials

Low pressure vinylation of aryl and vinyl halides via Heck–Mizoroki reactions using ethylene - ScienceDirect
Catalyst Assisted Selective Vinylation and Methylallylation of Quaternary Carbon Centre by using tert-Butyl Acetate | Catalysis | ChemRxiv | Cambridge Open Engage

PDF) Studies of microwave-enhanced Suzuki–Miyaura vinylation of electron-rich sterically hindered substrates utilizing potassium vinyltrifluoroborate | Stefan M Cooper, Jr. - Academia.edu

Transition‐Metal‐Free Deaminative Vinylation of Alkylamines - Hu - 2019 - Advanced Synthesis & Catalysis - Wiley Online Library